Ultrafast Light Controlled Cells
Cells that you can command with a flashlight
Programmed contracting protein network that is moving beads. Bead movement is tracked with blue dots.
can we make the cells in our body move according to our will?
In recent years, researchers have become increasingly interested in the questions that have thus far been science fiction—can we program living cells and use them as robots? If achieved, this breakthrough could revolutionize medicine, with applications ranging from cancer cell detection to targeted drug delivery within the human body.
But there are two big hurdles. First, most living cells are inherently complex, which makes controlling their movement a major challenge. Second, they rely on ATP for energy, which works like a single-use battery—once it’s consumed, it turns into ADP and can’t be reused.
Ca²⁺ diffuses into a sandwich-structured chamber from left to right, inducing a gel-like transition in the Tcb2 solution and contraction is happening along the boundary.
We’ve found a way around both problems using a type of simple microorganism called ciliates which can generate movement using calcium (Ca²⁺) ions instead of ATP. We’ve extracted a special protein from them, called Tcb2, and used it to build artificial protein networks in the lab. These networks reversibly contract and relax when we add or remove calcium, meaning we can actually control their movement. To take this even further, we’re using light to precisely guide the process. By leveraging commercially available calcium chelators—molecules that bind and release calcium when exposed to light—we can dynamically regulate calcium levels with a simple light switch. This enables us to reuse Ca²⁺ ions and direct the movement of our protein networks with high precision using spatial light patterns. The structure of these protein networks allows us to tweak their behaviors; by changing the protein density, we can create new movement patterns. We’ve created a mathematical model to describe these effects and used reinforcement learning to program precise motions. This breakthrough could lead to new smart materials for controlling tiny forces, building artificial cells, and creating responsive bio-materials.
Microscale protein network (right) contracting to a star pattern (left) within milliseconds.
Major questions
1) How does a non-ATP protein network move?
2) How can we finely manipulate how the protein network applies force at sub-cellular level?
3) How can we implement the protein network as a module for synthetic cells?
What we’ve discovered
TCB2 protein dynamics allow us to control the network with light and calcium.
Because the protein network exists inside of the cell, it is hard to study what forces influence it. We made a protocol to isolate protein, Tcb2, from Tetrahymena thermophila, to focus on the key properties of the protein. This protein naturally forms a fibrous network and contracts when exposed to calcium. Using light-sensitive chemicals to precisely control when and where calcium is released, we can control when and where the Tcb2 protein network contracts.
Pulsed light allows us to control the state of the network
To continuously “recharge” the system, we used a pulsed, or ratcheting, illumination protocol: by flashing light at the cells, the calcium-driven active region continues to grow and recruit Ca²⁺. To understand how the Tcb2 moves, we made a simulation to understand how the Tcb2 expands under different inputs. The pulsing allows us to repeatedly contract the network, maintaining sub-second contraction speeds, offering potential for Tcb2 networks to generate controlled micron-scale mechanical forces using light-based protocols.
The contracting protein network can be used to transport particles
By using simulations and reinforcement learning, we can generate a light pattern that controls the network to push or pull according to our will. The network can maneuver external objects such as liposomes, lipid particles, and polystyrene beads. In the near future, the protein network can be used to exert precise mechanical forces at the micrometer and second scales.
We use reinforcement learning to control the protein network
Using an artificial intelligence technique called reinforcement learning, we can develop protocols for precisely manipulating the motion of the Tcb2 gel. Here we show how the light intensity profile (shown in yellow) can dynamically control spatial densities of different molecules and contraction velocity of the gel.
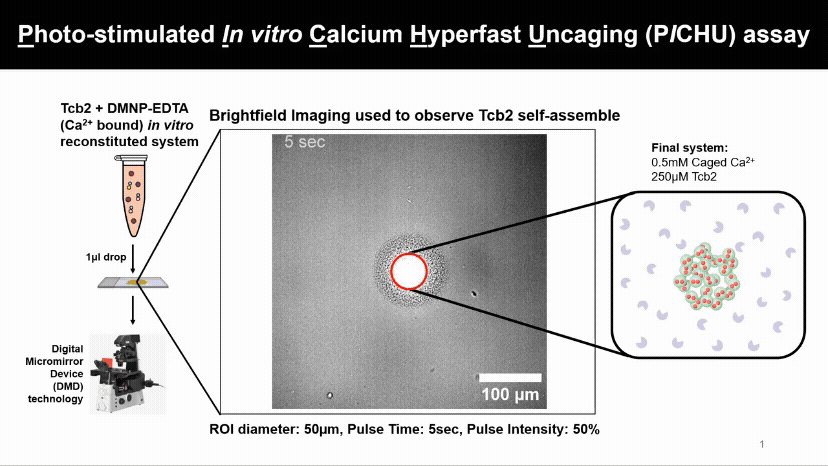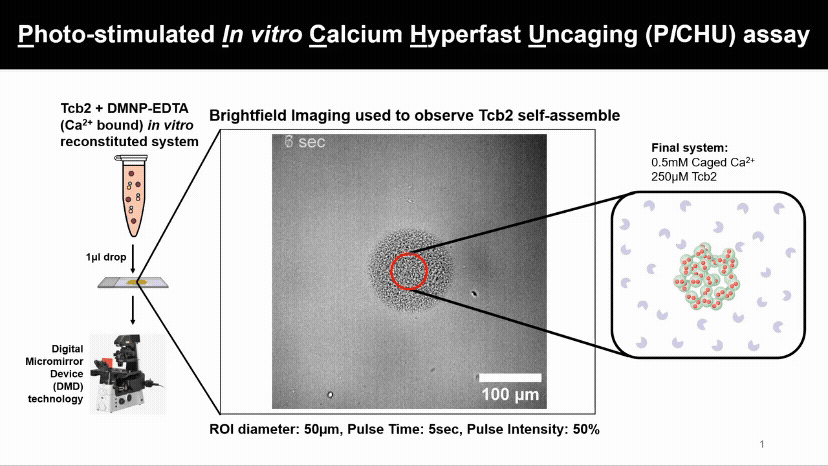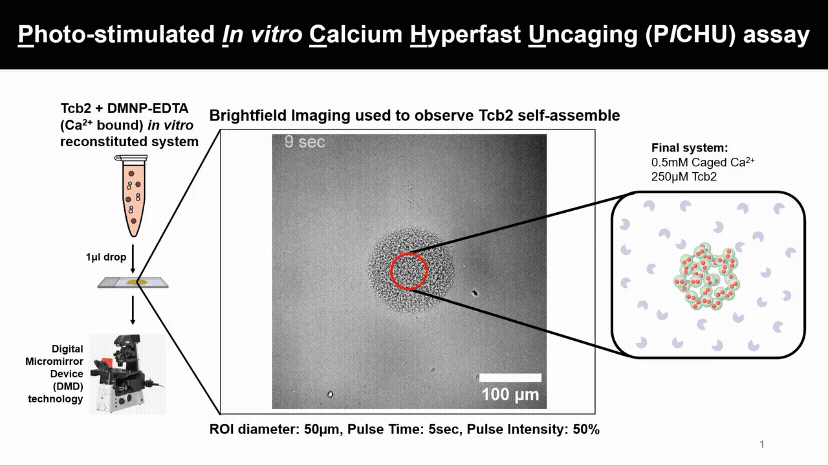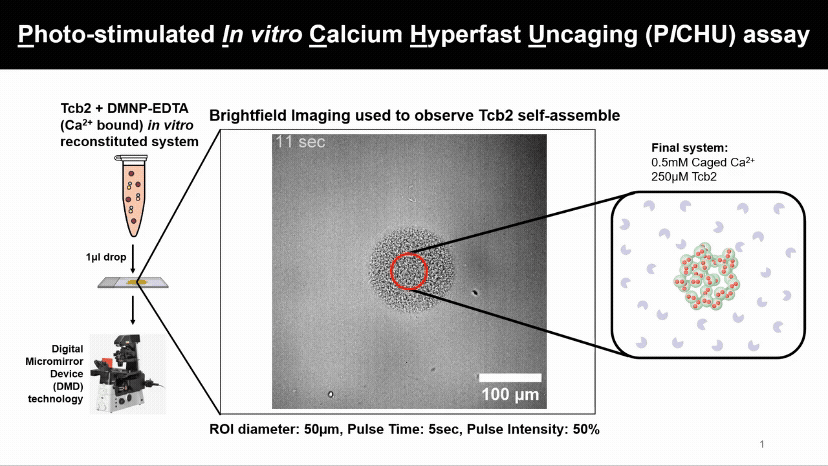
Light Controls Protein Assembly
A flash of light releasing calcium causes Tcb2 proteins to instantly build visible networks. This new assay lets us control when, where, and what ion levels triggers proteins assemble, revealing how light and ions can drive structure formation in real time.
Higher-order assemblies:
A single protein, like a biological LEGO piece, creates complex structures from just one building block. It's nature's instant architecture, triggered by calcium.
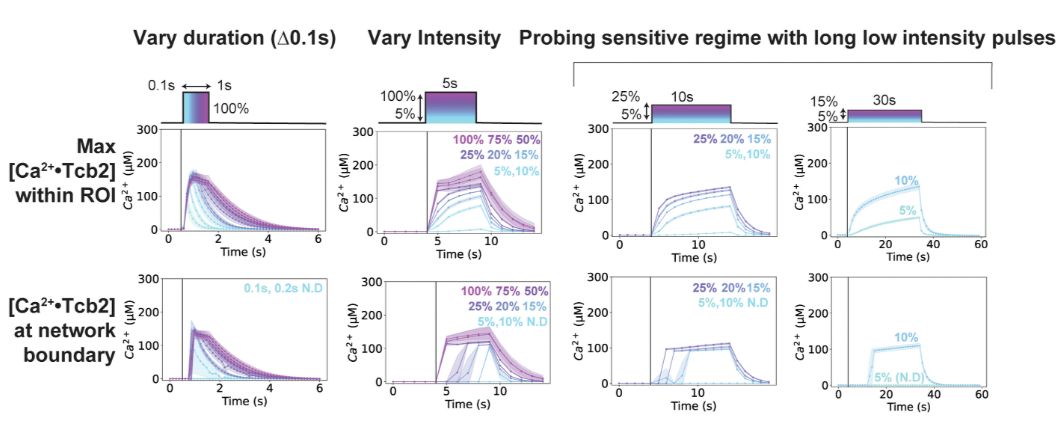
A Critical Calcium Level
We found that Tcb2 only assembles once calcium-bound monomers pass a critical level (~100 µM). Below that, nothing happens, but beyond it, the protein snaps into an organized network, showing a switch-like response to calcium.
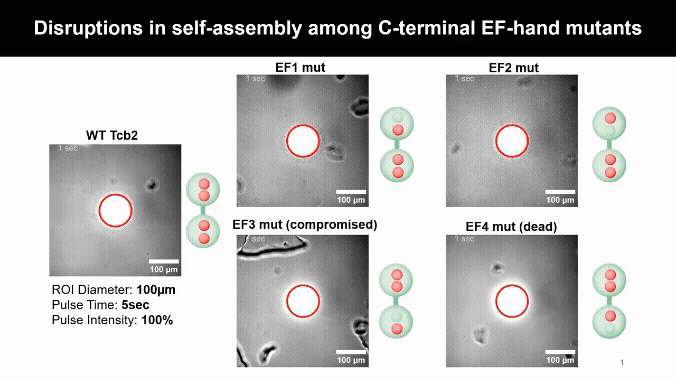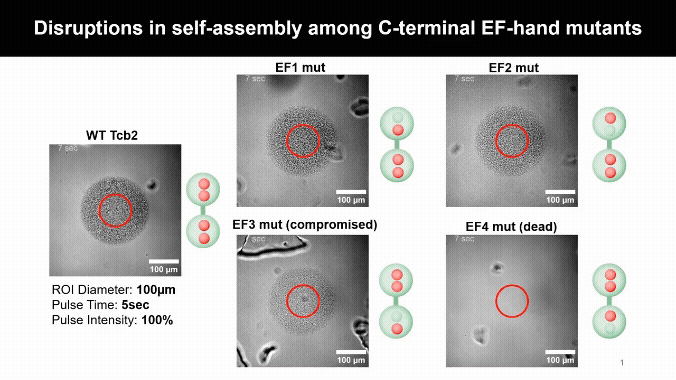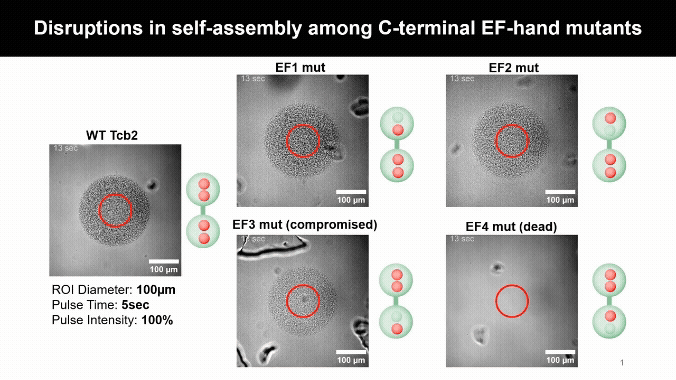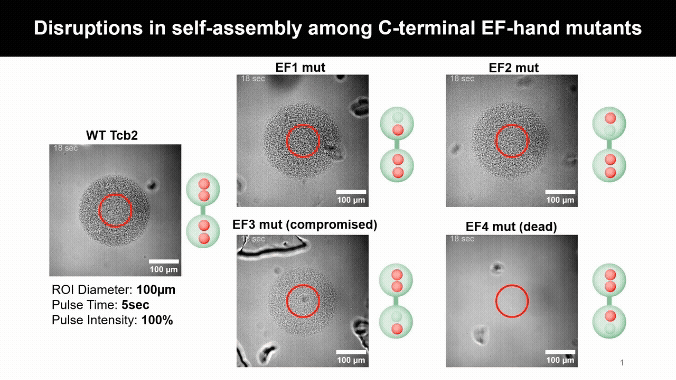
Mapping the Calcium Control Sites
Mutating Tcb2’s calcium-binding sites revealed one crucial residue, D184, that licenses self-assembly. Other sites simply tune how sensitive the protein is to calcium, uncovering a built-in molecular control system.
Read the papers
Light-induced reversible assembly and actuation in ultrafast Ca2+-driven chemomechanical protein networks. bioRxiv (2025).
Decoding ultrasensitive self-assembly of the calcium-regulated Tetrahymena cytoskeletal protein Tcb2 using optical actuation. bioRxiv (2025)